Project 3) Photocatalytic nanofibers made by electrospinning via sol-gel chemistry
One-dimensional (1D) nanostructures have superior charge transport properties, few grain boundaries, and especially metal oxide nanofibers prepared by electrospinning offer remarkable characteristics, e.g. high porosity and the highest surface-to-volume ratio in the class of 1D nanostructures [1,2]. Therefore, metal oxide nanofibers (or more general: ceramic nanofibers) are very interesting for highly efficient photocatalytic water splitting. Sol-gel chemistry is a low-cost solution-processing method for the preparation of photocatalytic materials and can be combined with the electrospinning process for the synthesis of ceramic nanofibers [2,3,4,5]. Figure 1 shows an SEM image of 3% Yttrium Partially Stabilized Zirconia (3YSZ) nanofibers [6].
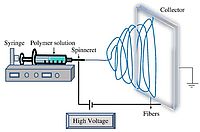
The electrospinning of nanofibers involves a relatively simple experimental setup, which allows the control over the diameter of the nanofibers and the possibility to synthesize continuous nanofibers that can have a length of several meters, but with a diameter of only a few nanometers [6]. Figure 2 shows a schematic overview of the electrospinning process. A high voltage (5 to 30 kV) is applied between a metallic collector and the needle of a syringe, which is filled with the precursor solution. As the solution is pumped through the syringe with a constant flow rate, a jet is ejected from the tip of the syringe, which moves towards the collector where the nanofibers are collected [7].
In this project, nanofibers of several interesting materials for photocatalytic water splitting are prepared by electrospinning. Using electrospinning, we are able to optimize many parameters, e.g. solution properties, processing parameters and the setup design, with which we can optimize the diameter, morphology as well as the alignment of the nanofibers in order to find the highest light-to-hydrogen efficiency. The photocatalytic properties of the obtained nanofibers will be investigated by photoelectrochemical (PEC) and gas chromatography (GC) measurements.
[1] A. C. Pradhan and T. Uyar, ACS Appl. Mater. Interfaces 2017, 9, 35757-35774.
[2] N. A. M. Barakat, K.-D. Woo, S.G. Ansari, J.-A. Ko, M. A. Kanjwal, and H. Y. Kim, Appl. Phys. A, 2009, 95 [3], 769-776. [3] W. Sigmund, J. Yuh, H. Park, V. Maneeratana, G. Pyrgiotakis, A. Daga, J. Taylor, and J. C. Nino, J. Am. Ceram. Soc. 2006, 89 [2], 395-407.
[4] M.S. Prévot, N. Guijarro, and K. Sivula, Chem. Sus. Chem. , 2015. 8 [8], 1359-1367.
[5] L.W. Shan, G.L. Wang, J. Suriyaprakash, D. Li, L.Z. Liu, and L.M. Dong, Journal of Alloys and Compounds, 2015, 636, 131-137.
[6] G. Cadafalch Gazquez, Electrospinning as a Tool for Fabricating Functional Ceramics, PhD thesis, 2016, University of Twente, Netherlands.
[7] Ning Zhu and Xiongbiao Chen (2013). Biofabrication of Tissue Scaffolds, Advances in Biomaterials Science and Biomedical Applications, Prof. Rosario Pignatello (Ed.), InTech, DOI: 10.5772/54125. Available from: www.intechopen.com/books/advances-in-biomaterials-science-and-biomedical-applications/biofabrication-of-tissue-scaffolds