Metal-Organic Frameworks
Metal-Organic Frameworks (MOFs) are a relatively new class of crystalline materials where metal ions or metal-oxo clusters and organic linkers are bound together in a highly ordered and microporous 3-dimensional network. These materials find application in many technologically important scientific areas such as gas separation and storage, lithium ion batteries, sensors and (photo)catalysis.
Our research focus includes the synthesis of MOFs (either in bulk or in the form of nanostructures), their characterization and their application in photo(electro)chemical hydrogen generation from water.
In particular, we use electrodeposition to deposit metal or metal oxide nanowires inside polycarbonate membranes used as templates. These metal or metal oxide nanowires are then converted into MOFs via electrochemical oxidation: During the oxidation, the metal is oxidized back into its respective cations, and as this reaction is performed in a solution containing the appropriate organic linker, the original nanowires are converted into MOF nanowires. After removal of the polycarbonate template, MOF nanowire arrays are obtained.
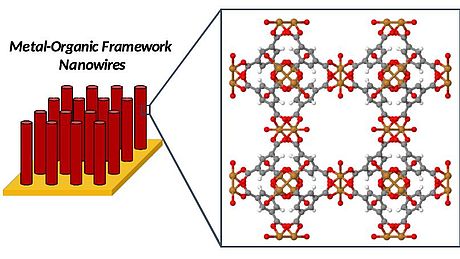
In another project, the complete process (the electrochemical deposition and electrochemical oxidation) for the preparation of MOF microstructures is developed without the use of templates. For this, we need to find the optimized electrochemical conditions to oxidize metallic microstructures into MOF microstructures while keeping their initial shape and size. After electrochemical oxidation, the desired final microstructure is a core-shell hybrid material, with the MOF as a coating on the metallic microcrystals.
We start with the electrodeposition of Cu2O microcrystals with pre-defined geometry. These microcrystals are electrochemically oxidized in a water-ethanol solution with the selected organic linker. By varying the electrochemical oxidation conditions as concentration, temperature, potential, etc., it is possible to change the final shape and size of the synthesized MOF crystals.
We optimized the process conditions used for the electrochemical oxidation of Cu2O microcrystals into Cu2(BTC)3, so that the microscale dimensions and geometry of the parent microstructure is maintained. It is important to maintain the initial geometry of the microstructure because we can study the photocatalytic properties of MOF microstructures and compare them with MOF powders and MOF thin films.
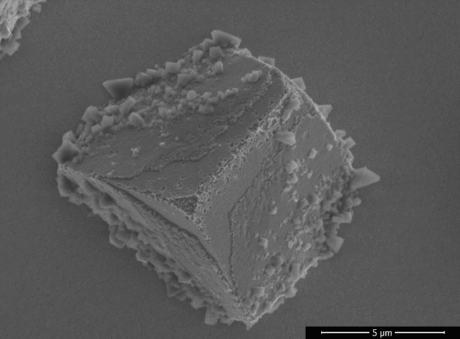
Both techniques enable the synthesis of MOF nanowires/microstructures as well as mixed composites with both metal oxide and MOF phases. These materials are highly promising for applications such as photo(electro)catalysis.




