Photocatalytic nanostructures
Metal oxides of abundant elements are of particular interest for photocatalytic water splitting because they are inexpensive, which makes their large-scale application possible. However, a disadvantage of these materials is their low minority charge carrier mobility and their relatively large penetration depth for light (50 nm and 3 ?m for CuBi2O4, respectively) [1]. In order to use these materials as electrodes for photocatalytic water splitting, a compromise must therefore be found between these two properties.
One possibility may be the use of quasi 1-D nanostructured electrodes. These have a long axis along the nanowires, which is well suited for light absorption. At the same time, the distance to the surface perpendicular to the axis of the nanowires is comparatively low, which allows the charge carriers to quickly reach the electrolyte/electrode interface.
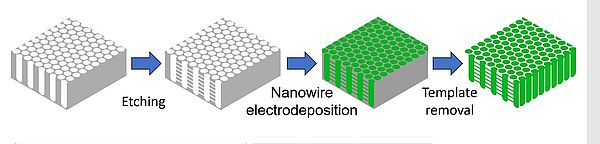
In order to enable a future large-scale use of the electrodes, electrochemical deposition is used as a cost-effective synthesis method. For this, porous alumina membranes with interconnected pores are filled with metal particles and later etched away, leaving a network of interconnected nanowires.
These networks have a lower light reflection and a much higher surface area than comparable thin-film electrodes and are therefore ideal for photocatalytic water splitting.
Literatur
[1] S.P. Berglund et al., Chemistry of Materials, vol. 28, no. 12, pp. 4231–4242, Jun. 2016.




